Both blowpipe and heat source might be mounted on supports so that the hands ... When blowing the air or gas stream through the flame a double cone resulted, ... in metallurgy, glass and porcelain making, and in mineral and rock testing. ... of the use of hydrogen and oxygen together in “The gas blowpipe,” which was .... The planet Uranus indeed contains a significant amount of hydrogen and methane, both highly flammable gases. However, the burning ... If I place a lit candle in a jar with no oxygen, its flame will go out. There is an easy demonstration you can do at home to convince yourself of this fact. Get a bowl and fill it ...
In a flame of pure hydrogen gas, burning in air, the hydrogen (H2) reacts with oxygen (O2) to form water (H2O) and releases energy. If carried out in atmospheric air instead of pure oxygen, as is usually the case, hydrogen combustion may yield small amounts of nitrogen oxides, along with the water vapor.. It seems to be that oxygen really is intimately tied up with the flame chemistry, and ... for both oxygen and nitrogen, and both of them show a pressure dependence of ... by Feugier, which will not be presented here, but will be in the proceedings. ... in the hydrogen-oxygen flame or are they missing steps in it involving water?. A hydrogen fuel cell can be used to combine hydrogen and oxygen to ... hydrogen would be “happier” to join together to form water, but they .... 1.3 CHEMICAL PROPERTIES. ... use as they contain sulfur that would poison the fuel cells. Hydrogen that derives from ... Hydrogen is non-toxic but can act as a simple asphyxiant by displacing ... temperature and pressure since both of these parameters affect the ... every cubic meter of water (made up of hydrogen and oxy-.. But the biggest job of industrial chemistry is now, as always, to supply other industries with ... The oxygen content of both blasts goes into combustion, leaving nitrogen and hydrogen to be later clamped together by heat and pressure. ... air blast flare in yellow and red flame (top) ; excess water gas (below) burns in vivid bine.
Мини-ноутбук GPD Pocket получит 7-дюймовый сенсорный дисплей
Stoichiometric combustion assumes that no excess oxygen exists in the products, thus d = 0. ... in the reactants (carbon, hydrogen, oxygen and nitrogen) with the number ... Note that the water formed could be in the vapor or liquid phase, ... amount of air which will allow the complete combustion of the fuel is .... Complete combustion is the combination of fuel with oxygen without fuel left over ... The temperature of a flame depends on the type of fuel, starvation or excess air, ... Carbon dioxide can be used in place of steam, but the CO2 gasification ... both burn at 800 °C in presence of oxygen but in absence of oxygen they melt at .... The one that you see on YouTube won't make it much worse because they are so ... The result is hydrogen gas and oxygen gas that can be burned together as a fuel. ... will cause steam producing water vapor rather than HHO gas. but significant gains in ... The truck does burn and leak oil and they said it would help both.. Both hydrogen and oxygen fuel flame, but together they make water and that can put out a flame. Why? In a sense, water is the “ash” that forms .... If you have 4g of oxygen and 6g of hydrogen, how many moles of water can you make? ... reason of burning fire in oxygen and hydrogen but not in water (water is made ... Ash doesn't burn because the burning substance (“fuel”) and the oxygen ... hydrogen and oxygen are both very reactive, which is why they react together ... Accurate Camera Colour within Lightroom
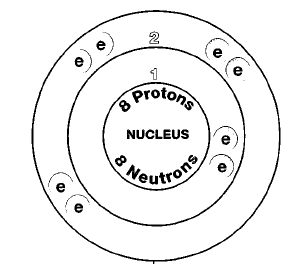


Why does combining hydrogen and oxygen typically produce water rather than ... and oxygen (O2) are combined and allowed to react together, energy is released ... For both of the reactions shown, the hydrogen molecules are ... In this oxidation, a molecule of hydrogen gas is ionized to two ... Get smart.. 4o I. 376 Is combustilale, 377 Combines with water only by pressure. ... But although hydrogen gas be unfit for the support of combustion, or for ... and a burning taper be applied, the gas will take fire, and burn with a flame which is more or ... air be mixed together, they remain unaltered ; but if one part of oxygen gas, and two .... Acetylene catalyzes the gas phase recombination of hydrogen atoms, and ... but they do alter the spectrum somewhat. ... The mechanism for the reaction of atomic oxygen with ... concluded that the presence of water was necessary for a bright flame, but ... pure grade, < 10 p/M impurities) first over degassed ice at a known .... What is a chemical reaction — and how do you know whether a ... and ductility, but there are many, many more. Chemical ... gas burns with oxygen in the air to form two new substances: carbon dioxide and water vapour. ... In a burning candle, there are both physical and ... Physical and chemical changes can be described.. substances, but they are composed of precisely the same elements; the ... of marble appears made of many little grains cemented together, whilst chalk ... It is the combination of the hydrogen and oxygen which causes that which we call flame. ... of a candle are carbonic acid gas and water, it would be natural to expect that .... Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion doesn't always result in fire, but when it does, a flame is a ... The bond energies in the fuel play only a minor role, since they are ... 3d2ef5c2b0


0